The CEOs of Eli Lilly and Novo Nordisk appeared alongside President Donald Trump at the White House last week to announce deals with the administration to cut prices for their blockbuster weight-loss drugs.
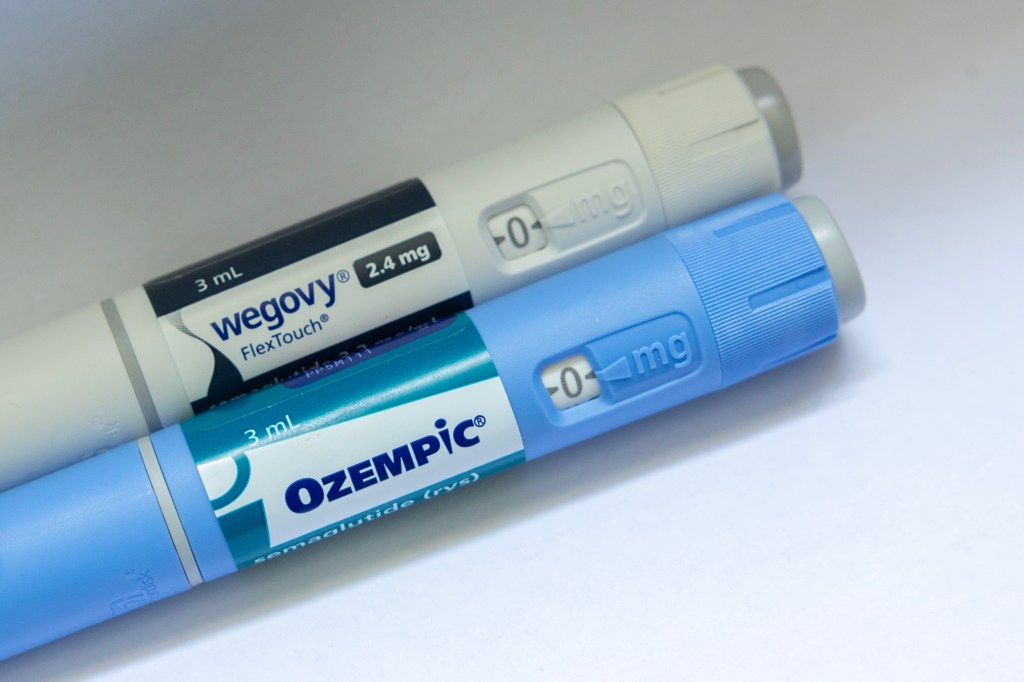
In exchange for the cuts, the Medicare program will offer coverage of Novo Nordisk’s Ozempic and Wegovy, and Eli Lilly’s Mounjaro and Zepbound, to millions of seniors. Currently, Medicare only covers the drugs, known collectively as GLP-1s, to treat diabetes and heart disease.
Originally developed to treat type 2 diabetes, GLP-1s have recently been approved to treat obesity. And while popular, they are not cheap.
According to reporting by the Wall Street Journal, GLP-1s currently cost consumers between about $1,000 and $1,350 for a month’s supply, though prices often come down once manufacturer rebates and discounts to insurers and cash-paying consumers are applied.
But many insurers still don’t cover the drugs to treat obesity. And despite the discounts announced last week, the prices can often prove cost-prohibitive for most Americans.
The Skinny on the deal
According to the terms of the deal, as reported by Axios, the two pharmaceutical giants will sell their weight-loss drugs to the Medicare programs for $245 a month to treat both diabetes and obesity. In turn, Medicare enrollees will be able to receive the drugs with just a co-pay of $50 a month as early as April 2026.
The deal also lowers the price of the drugs for state Medicaid programs, which could increase coverage for lower-income Americans.
Eli Lilly and Novo Nordisk also agreed to sell the drugs directly to consumers at an average price of $350 a month, with commitments to bring that down to about $250 over the next two years, according to the White House.
For its part, Indianapolis-based Eli Lilly will be off the hook for any new tariffs and drug-pricing programs introduced by the Trump administration, and Novo Nordisk, which is headquartered in Denmark, will receive a three-year tariff exemption.
Additionally, the Food and Drug Administration (FDA) will grant new, so-called Commissioner’s National Priority Vouchers to both companies to accelerate its review of their proposed new weight-loss pills.
“National priority vouchers are granted to a select group of products where the company has agreed to increase affordability, domesticate manufacturing as a national security issue, or address an unmet public health need,” said FDA Commissioner Marty Makary. “We are pioneering new ways of bringing these cures and meaningful treatments to the market faster.”
The companies have agreed to make those pills available for direct-to-consumer purchase at starting dose prices of $149 per month.
At the White House announcement, Health and Human Services Secretary Robert F Kennedy Jr. said: “These drugs have only been available for people who have wealth.”
But the deal still does little to cover everyone who’s eligible for the drug. Indeed, it does not apply to private insurance, and many insurers still do not cover GLP-1s for obesity treatment. Consequently, many patients purchase them out of their own pocket and will likely have to do so for the foreseeable future.
Additionally, it remains unclear if the lower costs the plan does provide will make the drugs affordable enough for those who aren’t insured.
Role reversal
In April, the Trump administration issued a Medicare D payment rule that prohibits coverage of weight-loss drugs, reversing a Biden administration-era proposal for Medicare to cover anti-obesity drugs, including GLP-1s.
According to recent reporting by Axios, the Biden plan, part of the former president’s signature Inflation Reduction Act (IRA), argued that reinterpreting the existing policy to cover the drugs for obesity would have made them more accessible to millions of Americans.
And while Medicare continues to negotiate the prices it will pay for Novo Nordisk’s Ozempic and Wegovy starting in 2027 under the IRA, the Trump administration’s deal is not part of that process.
A dose of skepticism
Some analysts are skeptical of whether the new deal between the pharmaceutical companies and the Trump administration is as big a deal as it is being touted as, or even if it was necessary at all.
“Is this a really big policy change, or is this a policy change that reflects ongoing policy changes in a competitive market? And that I don’t have a great answer to,” the American Enterprise Institute’s Ben Ippolito asked.
“I wouldn’t call it earth shattering, but it may be meaningful,” he added.
The lesson to compliance officers here seems to be that drug-pricing policy initiatives are still being ironed out in this Administration. So compliance officers should proceed accordingly.